Glow Stick Kinetics
Glow Stick Kinetics
Want to illuminate the concepts of kinetics? Try Glow Sticks! Glow sticks are a very popular accessory around Halloween because they are cheap, portable and they give off a ghostly glow. But do students understand the chemistry behind the plastic tube that gives them light?
The “glow” from a glow stick is the result of a process called chemiluminescence – a chemical reaction that provides the energy to emit light. A glow stick is housing for two separate chemical solutions. The outer plastic chamber contains a mixture of a phenyl oxalate ester and a fluorescent dye. The inner glass vial contains hydrogen peroxide. When the glow stick is bent, the glass vial breaks releasing the hydrogen peroxide and activating the reaction.
Students familiar with glow sticks will remember that the light fades over time. This fading provides a perfect backdrop for introducing students to kinetics.
Initial Investigation
To study the glow stick fading, you can use a Light Level Sensor. Create a reaction vessel using a Calorimeter Cup and some black electrical tape.

Tape the Calorimeter Cup to ensure a dark baseline. Leave a hole in the top for the Light Level Sensor.

Create a graph of Light Level vs. Time using SPARKvue® software. Remove the reactants from the plastic casing to eliminate interference with the Light Level Sensor.
CAUTION: The reactants are non-toxic and non-flammable, but contact with skin or eyes may cause discomfort. In case of contact, rinse with water. Reactants can also permanently stain clothing or furniture. Use appropriate precautions.
Carefully cut a hole in the top of the glow stick and pour the phenyl oxalate ester and fluorescent dye mixture into a small beaker. Then break the glass vial and pour the hydrogen peroxide into the small beaker.

Cover the reaction vessel with the lid and the Light Level Sensor, and start data collection.

Exploration
Now that it is established that the reaction can be studied with the Light Level Sensor, you can ask your students to explore factors that would affect the reaction. Some possible ideas for exploration could include:
- Can you make the reaction brighter?
- How does temperature affect the reaction?
- Does the concentration, or amount of reactants affect the reaction?
For example you could have the students look at temperature. Create a hot water bath. Cut the top off of the glow stick and heat it up in the hot water bath.

Once the hot water bath with the glow stick has reached a sufficient temperature (about 600C), pour some of the hot water into the Calorimeter Cup. Add a Fast Response Temperature Probe to the water in the Calorimeter Cup. Build a page with a graph of Light Level vs. Time and digits display of Temperature. Prepare the glow stick components as before and start data collection.

Using the Light Level Sensor, a Calorimeter and a glow stick provides a unique way to to explore kinetics with materials that are both familiar and fun!













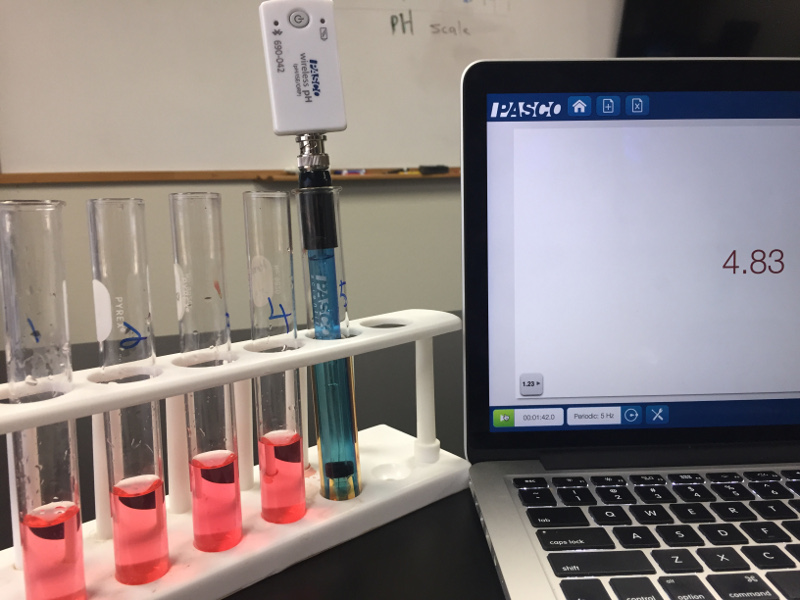
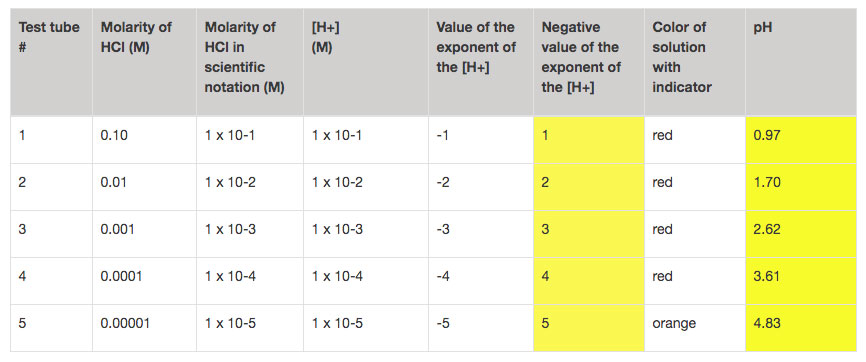
 Indicators are nice, but they really are just an indicator. In this case the indictor was not able to distinguish between the first four test tubes. (Note to self: get some new universal indicator!). Since the true colors aren’t shining through, it’s important to remember that to really understand pH, your students need to take actual pH measurements.
Indicators are nice, but they really are just an indicator. In this case the indictor was not able to distinguish between the first four test tubes. (Note to self: get some new universal indicator!). Since the true colors aren’t shining through, it’s important to remember that to really understand pH, your students need to take actual pH measurements.