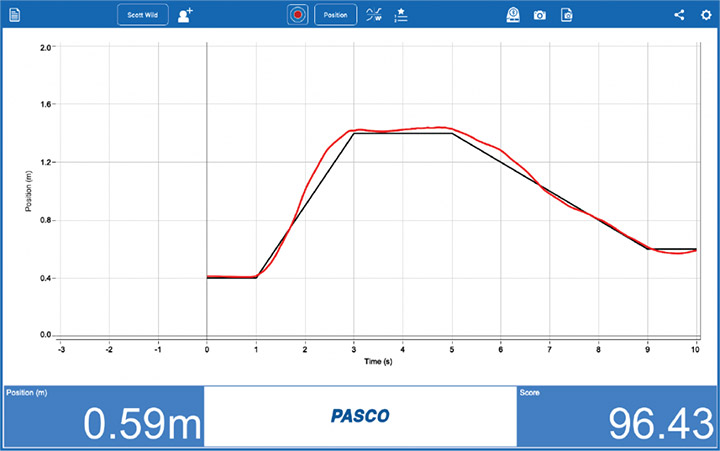
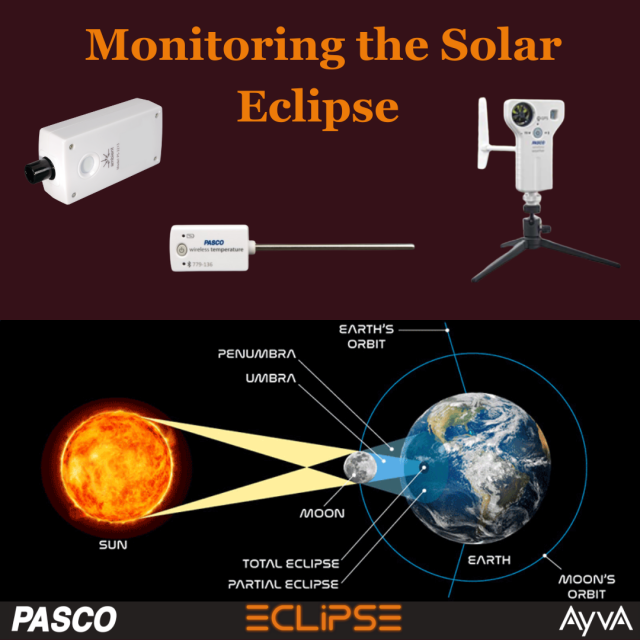
Monitoring the Solar Eclipse Using PASCO Sensors
Last Monday, on April 8th we were lucky enough to witness the rare phenomenon of a complete solar eclipse. Before the eclipse we set up many different PASCO sensors to see how it affects different environmental factors. We set up PASCOs Wireless Weather Sensor with GPS, Wireless Temperature Sensor, and Wireless Light & Colour Sensor.
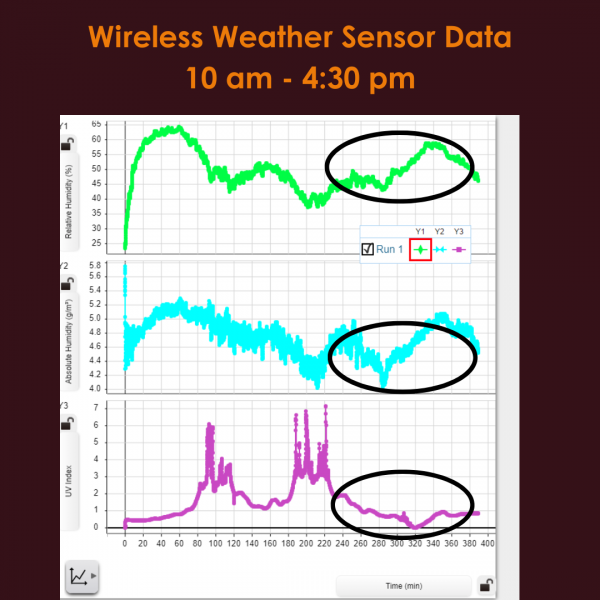
The weather sensor was set up to remote log from 10am until 4:30 pm at a sample rate of 1 second. After data collection we graphed the absolute humidity (g/m^2), relative humidity (%), and the UV Index in SPARKvue. As you can see around the time when the partial eclipse starts the UV index begins to fall and the relative humidity begins to increase. At the moment of complete totality UV index hits its minimum value of 0 and the relative humidity hits a maximum of 60%. Much like the UV Index absolute humidity begins decreasing at the time of the partial eclipse, however it hits its minimum value of 4.0 g/m^2 before the moment of complete totality.

Unlike the Wireless Weather Sensor both the Wireless Temperature and Light & Colour Sensors were set to remote log from 12 pm to 4:30 pm. The Wireless Temperature Sensor begins to increase from around 12- 2pm, at the beginning of the partial eclipse (140 on the X-axis) there is already a slow decrease down from a high of 14.6℃. The temperature reaches its lowest value 7.8℃ a few minutes after complete totality.
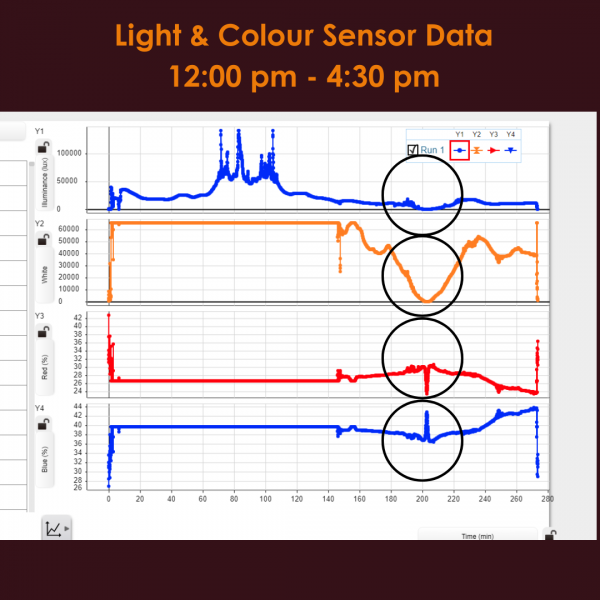
The Wireless Light & Colour Sensor showed some of the most interesting and instantaneous effects of the eclipse. Both red and blue light are rather consistent in their readings until the moment of totality where there is a sharp decrease in the % of red light and a Sharp Increase in the percentage of blue light. Both illuminance and white light follow a similar pattern of a parabolic shape, both reaching a minimum value of 0 at the moment of totality
Many thanks to PASCO sensors, whose contribution was integral to the success of this project! Their sensors enabled us to gather precise data effortlessly, minimizing the need for constant supervision. PASCO’s sensors serve as ideal educational tools for classrooms, helping students grasp fundamental concepts across physics, chemistry, and biology.
Featured Products:












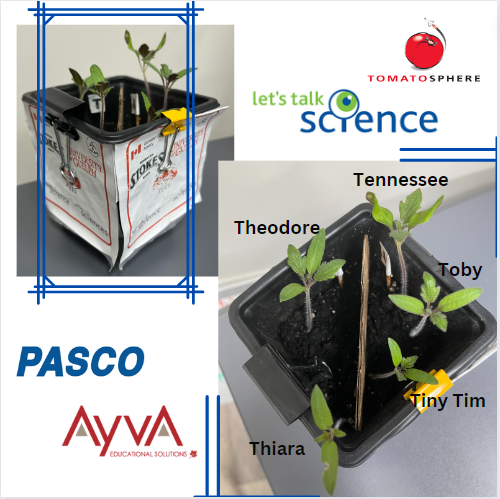 After just one week of being inside the Greenhouse, three out of six seeds germinated and sprouted! As a couple more weeks went by, two more seeds sprouted. Unfortunately, one seed (on the T side) did not germinate. Overall totaling three plants on the U side, two on the T side. At this point, we hypothesized which of the seeds had been to space and which had not, and wrote down our predictions to compare to the results later on. You can share your predictions in the survey at the bottom of this post, and find out which seeds were the space seeds!
After just one week of being inside the Greenhouse, three out of six seeds germinated and sprouted! As a couple more weeks went by, two more seeds sprouted. Unfortunately, one seed (on the T side) did not germinate. Overall totaling three plants on the U side, two on the T side. At this point, we hypothesized which of the seeds had been to space and which had not, and wrote down our predictions to compare to the results later on. You can share your predictions in the survey at the bottom of this post, and find out which seeds were the space seeds!