Under Pressure – Wireless sensor inside a balloon!
When introducing pressure and gas laws, I have always used balloons as a conceptual starting point because they are a familiar item to students. Unfortunately, I could not easily measure the actual pressure inside of the balloon— until now. The Wireless Pressure sensor make this possible and very easy. Pretty amazing right? What’s next, life on mars?
Even for those that are absolute beginners to pressure studies, this is a fun experiment, one that’s sure to garner you great fame among your students. Using a slightly larger balloon, and a little patience, the students who are able to get the pressure sensor into the balloon will definitely be heroes (if just for one day)
Now, with the sensor secured inside the balloon, let’s dance— or at least begin the experiment. Turn the sensor on and connect it to your device so your students can commence a countdown and start collecting data while they inflate the balloon!
The analysis tools in SPARKvue, allow the students to observe the pressure ch-ch-changes over time.
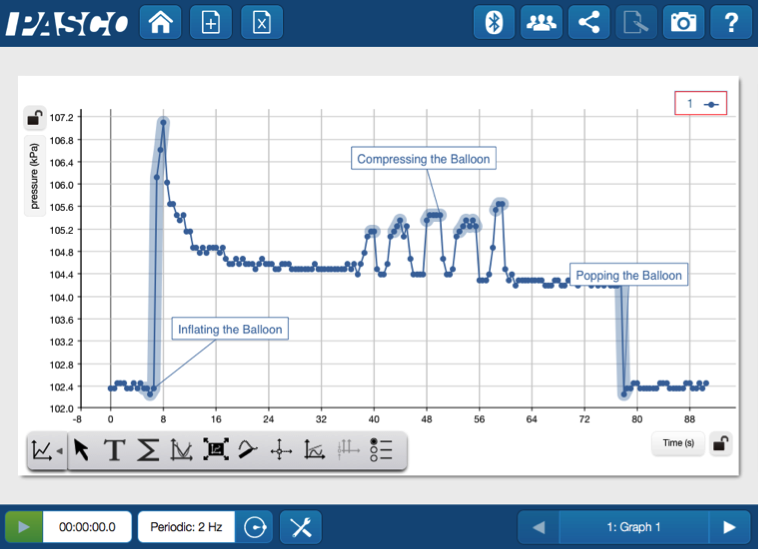
Some things to initially note could be the slight increase in pressure as the balloon is inflated, the changes in pressure as the balloon is compressed and the sharp decrease in pressure as when the balloon is popped. It’s a fantastic voyage through the many phases of the pressure change. Seeing data displayed in real time helps connect the science behind the sound and vision of a balloon popping.
Now that they have been engaged by the activities and explored the data (and before they start to act like a rebel, rebel) try having the students explain and elaborate on what is happening and why.
With this activity only taking a few minutes to complete it can be used as a demo, as a quick investigation or it can be grouped with a few other basic investigations where students move station to station to learn about gases. And as students begin to construct their knowledge about gas properties using familiar items, you can incorporate the empirical gas laws leading up to the ideal gas law.
To make this a truly memorable lesson, you and your students should be listening to “Under Pressure” by Queen and David Bowie while performing the activity. All the young dudes (and dudettes) will enjoy this one.