James Lincoln Introduces us to PASCO’s new Modular Circuits Kits
James Lincoln (physicsvideos.com) introduces teaches to PASCO’s new Modular Circuits Kits at NSTA 2017.
James Lincoln (physicsvideos.com) introduces teaches to PASCO’s new Modular Circuits Kits at NSTA 2017.
Students often struggle understanding pH. While we can tell them that it is a logarithmic function, students are more likely to associate “logs” with a calculator button or a piece of wood. So how do we get them to understand what the pH scale really means? Look for a lesson, instead of a pot of gold, at the end of a rainbow.
Let’s start with the acids. First have the students pour 10 mL of 0.1 M HCl into a test tube. Using graduated cylinders and pipets they can add 1 mL of that solution to another test tube with 9 mL of water making a 0.1 M solution. They should repeat the process of taking 1 mL of the previous solution and adding 9 mL of water until there are 5 solutions. They won’t know it, but they just performed a serial dilution. Now they can add some universal indictor to the solutions for a splash of colour.
 Indicators are nice, but they really are just an indicator. In this case the indictor was not able to distinguish between the first four test tubes. (Note to self: get some new universal indicator!). Since the true colors aren’t shining through, it’s important to remember that to really understand pH, your students need to take actual pH measurements.
Indicators are nice, but they really are just an indicator. In this case the indictor was not able to distinguish between the first four test tubes. (Note to self: get some new universal indicator!). Since the true colors aren’t shining through, it’s important to remember that to really understand pH, your students need to take actual pH measurements.
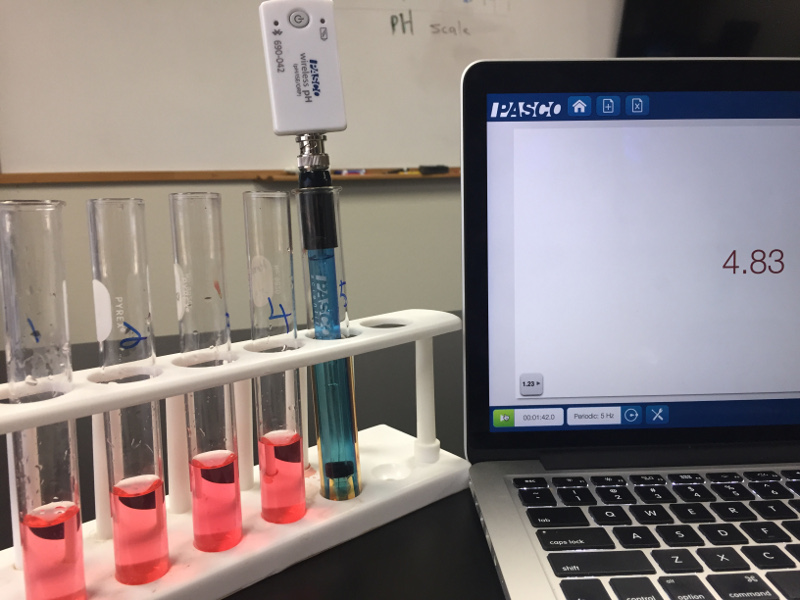
Now comes the pHun part! After recording the data for the solutions, it is important for students to try to make some meaning out of those measurements. Time to dust off those concentration calculation skills. They should be able to calculate the concentration, and write the concentration of the acids in scientific notation.
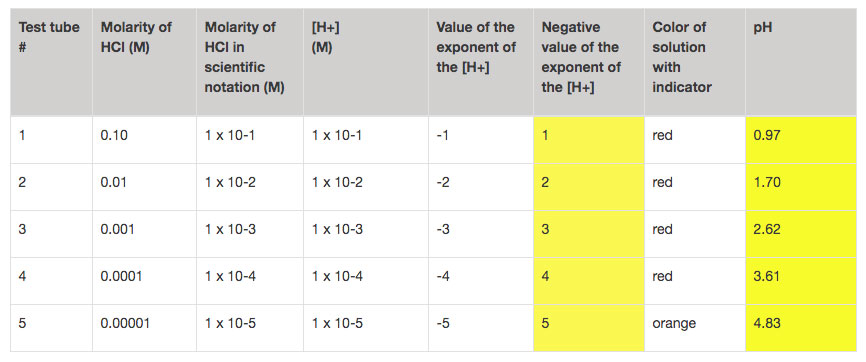
No need to travel somewhere over the rainbow, all your students need now are some good guiding questions and they should see that pH is primarily based on the negative exponent of the concentration of H+. With this understanding, pH=-log[H+] can be something more powerful than just a formula to plug and chug in calculator.
You can even extend this activity to pOH and its relationship to pH if you drop the base. Following the same procedure, students can perform a serial dilution starting with a 0.1 M NaOH solution.

After this colourful and engaging activity with the Wireless pH sensor and some fresh universal indicator, your students will be able to find the rainbow connection: a better understanding of the pH scale, what it means and how it’s measured.
It’s hard to believe that the end of the budget year is fast approaching. If your department has unspent funds now is a great time to consider acquiring one or more of PASCO’s premier instructional apparatus. The very popular featured products below are all in stock and can be shipped in time to make this year’s budget deadline.
1. Microwave Optics

The transmitter emits a large 3 cm wavelength that makes it easy for students to visualize and understand electromagnetic interactions. The system can be quickly adjusted with magnetic mounting components, rotatable transmitters and receivers and a Goniometer with rotatable arms featuring built-in degree and millimeter scales. Durably designed, the system will provide years of trouble free labs with components made of either cast-die aluminum or stainless steel.
WA-9314C ($2995) – Basic System for investigation electromagnetic interactions
WA-9316A ($3995) – Advanced System includes accessories for Brewster Angle and Bragg Diffraction experiments

This very versatile system’s open design is ideal for education. When used with Capstone software, students can graph the spectral lines of gases; precisely measure the relationship between angle wave length and intensity; and analyze the transmission characteristics of filters and chemical solutions. The sensors can connect to PASCO’s full range of interfaces including the very affordable Wireless Airlink or the powerful 850 universal interface.
OS-8450 ($1912) – Includes Light and Rotary Motion Sensors and Optics Bench
OS-8537 ($1257) – Sensors and Optics Bench not included

Planck’s constant is a central quantity in quantum mechanics and its discovery was one of the greatest breakthroughs in understanding the nature of light. With this system your students will be able to perform the photoelectric experiment to determine Planck’s Constant to within 5%. Students will also be able to verify that stopping voltage is independent of intensity and find the characteristics of the photodiode. Can be used with the 850 Interface and Capstone software
SE-6614 ($3156) – Basic System, includes Mercury Light Source with Hg tube
SE-6609 ($5666) – Basic System plus DC Current Amplifier and DC Power Supply

This system reproduces J.J Thompson’s landmark experiment to calculate the charge-to-mass ratio of the electron. A very sharp and visible electron beam within the vacuum tube allows for its radius (R) to be easily measured using the built-in fluorescent scale. The system also provides a measurement for the accelerating potential (V) applied to the electron gun as well as the magnetic field (B) produced from applying a current to the Helmholtz coils. With these measurements students can then accurately calculate the electron’s charge to mass ratio using the formula e/m=2V/B2R2.
SE-9629 ($6555) – Complete system with e/m tube and power supplies
The ability to easily and affordably measure CO2 levels is great news for Biology and Environmental Science teachers.

PASCO’s new Wireless CO2 Sensor communicates directly with a wide range of Bluetooth equipped computers without requiring an expensive interface.
Ambient environmental Carbon Dioxide levels are typically very low. This means any experimental changes to CO2 levels tend to be significant in percentage terms providing convincing and reliable evidence of the phenomenon being studied.
Investigations based on the wireless CO2 sensors are easy to setup and they work.
Other opportunities for CO2 investigations include:
I have taught grade 9 applied science, science and technology, grade 10 applied, regular and enriched science, grade 11 chemistry and physics for 33 years at Westwood Senior High School in Hudson Québec. I discovered the PASCO equipment in 2019 and it completely changed my life. I love to discover, produce experiments and share discoveries. I am looking forward to work with your team.
Having graduated with a major in Computer Science and minors in Physics and Mathematics, I began my teaching career at Killarney Collegiate Institute in Killarney, Manitoba in 2009. While teaching Physics there, I decided to invest in PASCO products and approached the Killarney Foundation with a proposal about funding the Physics lab with the SPARK Science Learning System and sensors. While there I also started a tremendously successful new course that gave students the ability to explore their interests in science and consisted of students completing one project a month, two of which were to be hands-on experiments, two of which were to be research based, and the final being up to the student.
In 2011 I moved back to Brandon, Manitoba and started working at the school I had graduated from, Crocus Plains Regional Secondary School. In 2018 I finally had the opportunity to once again teach Physics and have been working hard to build the program. Being in the vocational school for the region has led to many opportunities to collaborate with our Electronics, Design Drafting, Welding, and Photography departments on highly engaging inter-disciplinary projects. I believe very strongly in showing students what Physics can look like and build lots of demonstrations and experiments for my classes to use, including a Reuben’s tube, an electromagnetic ring launcher, and Schlieren optics setup, just to name a few that have become fan favourites among the students in our building. At the end of my first year teaching Physics at Crocus Plains I applied for CERN’s International High School Teacher Programme and became the first Canadian selected through direct entry in the 21 years of the program. This incredible opportunity gave me the opportunity to learn from scientists working on the Large Hadron Collider and from CERN’s educational outreach team at the S’Cool Lab. Following this, I returned to Canada and began working with the Perimeter Institute, becoming part of their Teacher Network.
These experiences and being part of professional development workshops with the AAPT and the Canadian Light Source (CLS) this summer has given me the opportunity to speak to many Physics educators around the world to gain new insights into how my classroom evolves. As I work to build our program, I am exploring new ideas that see students take an active role in their learning, more inter-disciplinary work with departments in our school, the development of a STEM For Girls program in our building, and organizing participation in challenges from the ESA, the Students on the Beamline program from CLS, and our local science fair.
Though I graduated with a BEd qualified to teach English and Social Studies, it just wasn’t meant to be. My first job was teaching technology courses at a local high school, a far cry from the English and Social Studies job I had envisioned myself in. I was lucky enough to stay in that position for over ten years, teaching various technology courses in grades 10-12, while also obtaining a Master of Education in Technology Integration and a Master of Education in Online Instructional Media.
You will notice what is absent from my bio is any background in science. In fact, I took the minimum amount of required science courses to graduate high school. Three years ago I switched roles and currently work as a Technology Integration Leader; supporting teachers with integrating technology into their pedagogy in connection with the provincial outcomes. All of our schools have PASCO sensors at some level (mostly grades 4-12) and I made it my professional goal to not only learn how to use them, but to find ways to make them more approachable for teachers with no formal science background (like me!). Having no background or training in science has allowed me to experience a renewed love of Science, making it easier for me to support teachers in learning how to use PASCO sensors in their classrooms. I wholeheartedly believe that if more teachers could see just how easy they are to use, the more they will use them in the classroom and I’ve made it my goal to do exactly that.
I enjoy coming up with out-of-the-box ways of using the sensors, including finding curriculum connections within subjects outside of the typical science realm. I have found that hands on activities with immediate feedback, which PASCO sensors provide, help students and teachers see the benefits of technology in the classroom and will help more students foster a love of science and STEAM learning.