Concentrate on the Conductivity
Students have a hard time concentrating when the topic is solution concentration. The solution may be having them create their own solutions.
Using some solid solutes and glassware, have them make a solution with a specific molarity. They should write down their own steps and follow their procedure. Make sure they are specific with the type of glassware and the quantities of solute and solvent. This is a good way for them to review items or steps that they may have forgotten and to put theory into practice.
Have them make two solutions of the same molarity: one from an ionic solute and the other from a covalent solute. After revising their procedure and correctly massing their solutes, you can take stock of their work, while they create stock solutions. Add a splash of food coloring to each solution to help them distinguish between the two. There is a good pedagogical reason, and an added bonus that a dash of color makes any activity more festive!
After making up their stock solutions from the solid solutes, they can concentrate on the concentration even more by making dilute solutions. They should create a series of dilutions that are the same volume. Before they start pouring, make sure they understand mole and volume relationships by have them write out their calculations and procedures.
Those drops of food dye will really come in handy as they prepare the solutions because they’ll serve as a visual check on the concentration! They can see that the solution is getting more dilute, and you can quickly see if they are on the right track.
Now it’s time to kick this solution investigation up a notch. They can measure the conductivity of the solutions using SPARKvue and the Wireless Conductivity Sensor.
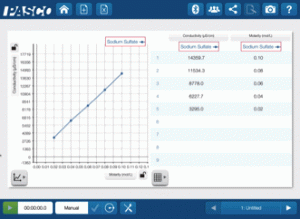
First have them test the solutions with the ionic compound solute. As they collect the data, they should plot Conductivity vs. Concentration.
There are a couple of key concepts that they can quickly discern from this graph. The solutions containing the ionic compound are definitely electrolytic (they conduct electricity), and the conductivity is directly related to the concentration.
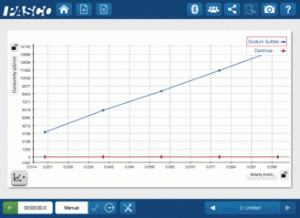
What happens with the covalent compound solutions? Will they see the same relationship?
There is a straight line on the graph for the covalent compound— it just happens to be flat and approximately zero for all the concentrations! Even though they could see from the dyes that the solutions were different, the conductivity did not change. But why? These solutions are non-electrolytic. No matter how high or low the concentration, the conductivity will not be affected.
Through this activity, students get to concentrate on concentration by making and testing their own solutions. The food coloring and the Wireless Conductivity Sensor provide visual cues and quantitative data as the students construct their own cognitive concentration concepts (say that five times fast). Even a measurement of zero can be important because, in this case, it can drive home the important difference between electrolytes and non-electrolytes.