Students are familiar with the concept of weather – they likely use an app to see what it’s going to be like every morning to decide what to wear. But do they know the difference between weather and climate? How can we help them understand that weather refers to local conditions over a short period of time while climate identifies atmospheric behavior over longer periods of time?
We can start by having them measure “what’s happening in your neck of the woods”. The question shouldn’t be “What is THE weather like today?” Instead it should be, “What is YOUR weather like today?”
This change in context can help them understand that weather is local. The conditions that they experience could be very different not only from what someone experiences across the country, but even from what the weather is like just a few miles away.
Using the Wireless Weather Sensor with GPS you can measure and monitor local weather conditions. Simply take it outside, connect to SPARKvue, and start collecting data.

The sensor is capable of making 17 different measurements. To keep the data collection focused, you can set up a display to make the measurements look like a dashboard for your own personal (temporary) weather station
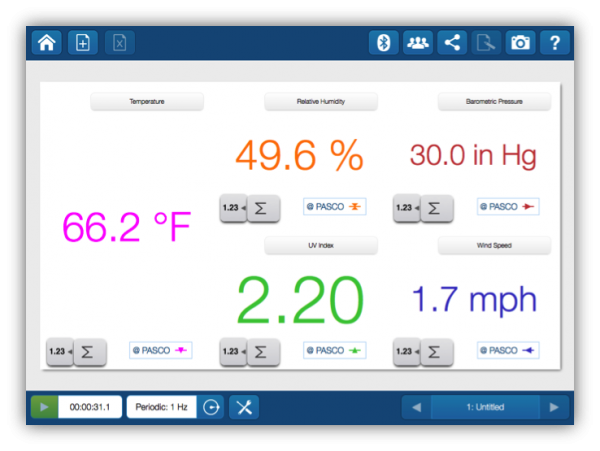
In SPARKvue you can change the units to match the units that are reported on actual weather stations. For example, in science we typically measure temperature in degrees Celsius, but weather in the US is reported in degrees Fahrenheit. This provides a good opportunity to talk about measurement units and how they are related.
Once the students collect “their” weather data, they can check that against the forecasted weather for the area at the same time.
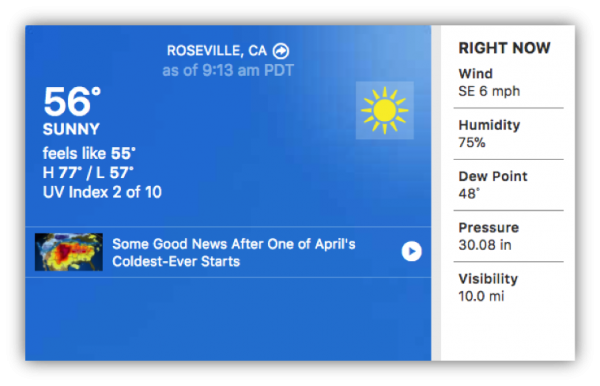
To get a broader perspective, students can compare “their” weather to conditions in other places at the same time. For instance, PASCO scientific is at a latitude of about 38.8 degrees north. Across the country, at about the same latitude, but a very different longitude, lies Washington, D.C.
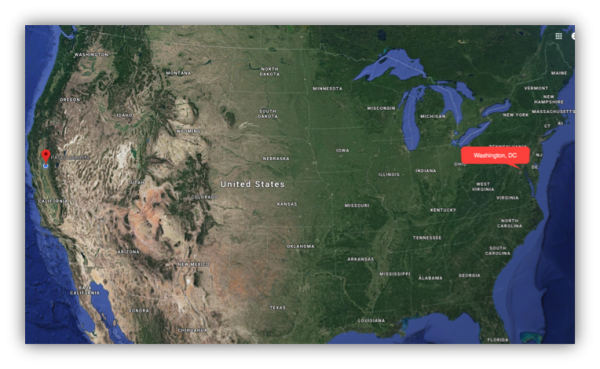
The change in longitude, going from the West Coast to the East Coast, can mean very different weather.
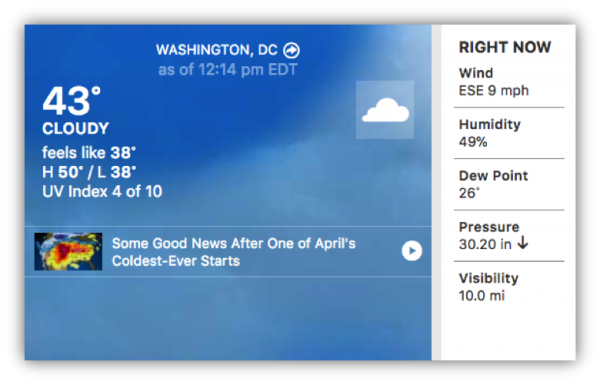
Your students don’t have to travel across the country to see differences in weather. Having multiple students collect data at different areas around the school or home provides a great opportunity to analyze data and incorporate science and engineering practices into your lesson. They can analyze and interpret the data by comparing both to each other’s data from different locations around the school, and to local and remote weather station data on the same day at the same time.
Using the Wireless Weather Sensor with GPS, students can not only collect data across a range of locations but also over periods of time. Weather can change from minute-to-minute, hour-to-hour, and season-to-season. As they look at averages over longer and longer periods of time, they are really beginning to look at how the climate is changing – not just short-term weather phenomena. To appreciate the difference between weather and climate, they would need to do some additional research and look at long-term historical weather data for their area.
Next time your students ask about THE weather, use the Wireless Weather sensor to collect some data so they can collect evidence about THEIR weather.
Related Products:
Wireless Weather Sensor with GPS (PS-3209)
SPARKvue Single User License (PS-2401)